Carbon capture and storage
A new, integrated, approach to mineralisation-based CCS
1 November 2009Mineralisation, ie conversion of CO2 to carbonates, similar to those that occur in nature, is an attractive concept for carbon capture and storage as it can avoid some of the potential public acceptance problems of geosequestering pressurised CO2. But there are major cost and energy penalty concerns. A new integrated approach to mineralisation, avoiding the intermediate step of CO2 extraction and promising considerable benefits, is outlined here.
The ICS (Integrated Carbon Sequestration) process envisages a new approach to mineralisation-based carbon capture and storage. Carbon dioxide is captured from power station flue gases and converted to mineral carbonates. These are benign and geologically stable and their carbon dioxide content is auditable in perpetuity. But the process also differs from most other CCS processes, including previously proposed mineralisation schemes, in that it is integrated and avoids any need to handle carbon dioxide as a pure gas, reducing energy consumption and estimated costs.
The existing coal fleet: what’s to be done?
The ICS process also effectively addresses the current reality of coal-fired power generation: the difficulty of prematurely shutting down power stations in operation, under construction or at an advanced stage of planning. By 2020 they will be producing around 10 billion tonnes of carbon dioxide annually. If proposed limits (now set at 450 parts per million but with calls for even lower levels) are to be met for atmospheric concentrations of this gas, almost all of it must be captured from flue gases and stored somewhere, safely and permanently. Almost all existing and currently proposed power stations rely on pulverised-fuel combustion, so capping atmospheric concentrations of carbon dioxide demands above all, that as many as practicable of the world’s fleet of coal-fired power stations be fitted or retrofitted with post-combustion carbon capture and storage plant.
For carbon capture, designers of plant intending to do this are able to draw upon decades of experience with the sweetening of raw natural gas, although the challenges involved in extending these designs to the much larger volumes of flue gases from coal-fired power stations are daunting.
One is particularly intractable: the substantial energy requirements, both to strip carbon dioxide in an acceptably pure form from the flue gas scrubbing media so that these may be returned to the process, and to compress the gas as needed for its mostly long-distance transport by pipeline and subsequent storage.
The large energy requirements arise essentially because all of the approaches currently favoured for carbon dioxide storage involve geosequestration, ie, forcing the gas under pressure deep underground into un-mineable coal seams, depleted oil and gas fields, or saline aquifers.
Even with further development, stripping and compression alone promise to absorb at least one quarter of the energy released from the coal burned in the host power stations’ boilers. Adding other losses, to maintain their net electricity exports generators can expect to see their coal consumption rise by half as much again, just to supply the additional energy required to operate the post-combustion capture and gas compression systems required for their power stations to qualify as low-emission.*
Geosequestration presents further challenges, none greater than the need to evaluate repositories in sufficient detail to address public and regulatory concerns, including their certification by some competent, independent authority to be completely secure against leaks, tremors or groundwater acidification, essentially for ever.
The ‘acceptance bar’ will be set high, so the process of evaluating and certifying every repository will be slow and expensive, to the point where this will probably determine how fast carbon capture and storage by geosequestration can be deployed worldwide. This is geosequestration’s Achilles’ heel.
For this reason alone, few can be comforted that geosequestration will, on current projections, be the only horse running in the carbon storage stakes.
A second horse: mineralisation
An entirely natural process, mineralisation, has long been regarded as the basis for a possible alternative to geosequestration. The term refers to part of the natural geological carbon cycle, where certain silicate rocks (in particular the ultramafics, which are igneous rocks that have a particularly high ratio of magnesium to silica), when exposed to the atmospheric elements, weather to produce carbonates, mostly magnesite (magnesium carbonate).
Such ultramafics are abundant: the Earth’s upper mantle is formed mainly of peridotite, a rock comprised of the minerals olivine and pyroxene. Being dense and prone to quite rapid (in geological terms) weathering, such primary ultramafics tend to be restricted to the planet’s more geologically active regions including the eastern Mediterranean (from Italy through to the Middle East), western North America, Japan, New Zealand, and other countries along the Pacific Ring of Fire.
If such rocks are first infiltrated by water as they are where they lie beneath the oceans, they may combine chemically with some of this water to form serpentinites, another family of ultramafic rocks comprised mainly of three minerals: antigorite, lizardite and chrysotile (of which a rare but troublesome form is white asbestos). The serpentinites are more resistant to weathering, so are more abundant in the Earth’s crust, being found even within and around the margins of geologically ancient shields such as those in Australia, Canada and South Africa. They are present in all continents in quantities that far exceed the total needed to absorb the carbon dioxide emitted from all of the world’s power stations for centuries to come. The Antigorio Valley in Italy gives its name to antigorite, the Lizard Peninsula in Cornwall, UK, to lizardite. The State Rock of California is serpentinite.
The conversion of these rocks to carbonates completes, in the thermodynamic sense, the oxidation of carbon: heat is released when the carbon in coal or other organic matter burns or is otherwise oxidised to form carbon dioxide; more heat is released when the carbon dioxide reacts with silicate minerals in ultramafic rocks to form carbonates (and silica). Being thermodynamically favoured, the carbonation reactions can only be reversed by subjecting these carbonates to bright red heat, as occurs in nature should they be subducted deep into the Earth’s mantle, whence they yield up their carbon dioxide and water (as steam). These gases may eventually (tens if not hundreds of millions of years after they were first captured from the atmosphere) work their way back to the surface via volcanoes.
The natural weathering of ultramafic rocks has been occurring for as long as there has been water and carbon dioxide in the atmosphere. Had it not occurred, Earth would have an atmosphere like that of the planet Venus, which is around 95 times as dense as ours, at ground level has a temperature of around 485°C, and is 96 per cent carbon dioxide.
The obvious problem with mineralisation as it occurs in nature is that the reactions, while fast in geological terms, are far too slow to form the basis of a practicable ex situ (in a reactor vessel) carbonation process. There have been many attempts at bench scale to speed up these reactions, arguably the most practicable (of those where results have been published) being those undertaken around 1999/2000 at the US Department of Energy’s Albany (Washington state) laboratories. There, by subjecting heat-treated, finely ground antigorite suspended in an aqueous solution of salt and sodium bicarbonate to carbon dioxide under high temperatures and pressures (more than 100 bar), researchers were able to achieve conversions of magnesium silicates to carbonates of close to 80 per cent in half an hour (see, for example, http://www.osti.gov/bridge/servlets/purl/896218-YSyk5m/896218.PDF).
This approach to mineralisation therefore shares, with geosequestration, the need to start with highly pressurised carbon dioxide, so must similarly incur the energy penalties associated with stripping the gas from the solutions or other media used to capture it from flue gases, then compressing it.
The second obvious problem with mineralisation processes is that in practical terms, the storage of one tonne of carbon dioxide requires around three tonnes of serpentinite or equivalent ultramafic rock – or expressed another way, 6-7 tonnes of such rocks are required to absorb the carbon dioxide from the combustion of every tonne of coal. This rock must be quarried, transported, pre-treated either by heating or fine grinding, or both. Then, once carbonated, the rock must be emplaced safely and permanently.
The additional cost and energy consumption involved in these activities put this two-step ex situ approach to mineralisation at such a competitive disadvantage that most interest in it has fallen away. Interest in mineralisation seems now to be restricted to in situ processes, ie the injecting of pressurised carbon dioxide into ultramafic-rock-rich geological formations such as those that exist for example in Oman and Iceland.
Mineralisation revisited
The ICS process uses mineralisation, but in a new way, and, seemingly alone amongst the CCS processes currently being developed around the world, avoids the need to handle carbon dioxide as a pure gas.
Furthermore, the energy balance of the total process gains from the heat liberated by the mineral carbonation reactions. The ICS process promises the advantages of mineralisation for secure, permanent carbon storage without many of the cost and energy penalties associated with two-step mineralisation processes; it is an integrated process.
After extensive literature searches confirmed its novelty, and a considerable amount of laboratory work confirmed in practice the key reactions, patent applications have been lodged in all major coal producing and consuming countries.
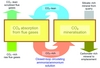
In its simplest form, as shown in Figure 1, the ICS process integrates two basic operations: in the first, an ammonia-rich aqueous solution of ammonium bicarbonate is used to scrub carbon dioxide from flue gases to form a solution enriched in carbon dioxide. In the second, pre-treated serpentinite or other suitable ultramafic rock is blended directly into this rich solution prior to the mixture being held under controlled conditions in a purpose-built reactor. There, via what is at first glance a double decomposition reaction, the silicate minerals react with ammonium carbonate to form magnesium carbonate (magnesite) and silica, thereby directly removing from solution the carbon dioxide that was absorbed in the flue gas scrubbers, as insoluble precipitates.
By simply filtering or otherwise separating out the insoluble solids, the capture solution is regenerated to the extent necessary for its recycle to the flue gas scrubbers. Under the correct conditions ammonia has a catalytic role; it is not consumed in the process. Ammonia losses are restricted to residual quantities accompanying the final carbonated mineral, and as ammonium sulphates and nitrate formed as a consequence of the scrubbing of oxides of sulphur and nitrogen (SOx and NOx) in the raw flue gases. The ICS process embraces optional further steps for recovering these residual quantities of ammonia, and plant for keeping ammonia slip in the final flue gases below one part per million.
Over the last couple of years, a group of scientists from CSIRO (Australia’s major national government research body, www.csiro.au) contracted to ICS have been conducting autoclave trials at Lucas Heights south of Sydney. These trials have demonstrated “proof of concept”, notably the direct conversion of silicates to magnesite, and work continues to establish optimum conditions for this reaction to allow a continuous pilot plant to be designed. Their thermodynamic modelling has confirmed the energy efficiency of the process.
Pre-feasibility studies into the application of the ICS process at large scale, eg, retrofitting it to major existing coal-fired power stations to capture at least 90% of their carbon dioxide emissions, suggest a total cost of US$50 to capture and store each tonne of the gas permanently and securely as emplaced mineral carbonate.
Since most existing power stations may normally be expected to lie some distance from suitable silicate rock deposits, such estimates must include allowances for transporting the rock to the power stations and for the transport of the carbonated mineral. The figure of US$50/tonne includes an allowance for 200 kilometres of rock transport by rail, plus some final carbonated mineral transport by slurry pipelines.
The above summarises the position for existing power stations. But since the tonnage of rock required for this mineralisation process is six times the tonnage of coal to be fired in the host power station, logic dictates that a new power station would be built nearer the rock deposit than the coal field that furnishes its fuel. Under these conditions the cost of capturing and permanently storing by mineralisation one tonne of carbon dioxide may reasonably be expected to fall below US $40/tonne of the gas. (All estimates, capital and operating, include contingencies.)
Mention was made earlier of geosequestration’s Achilles heel: that its rate of deployment will inevitably be held hostage to the slow and expensive process of locating, characterising and certifying repositories for pressurised carbon dioxide. In contrast, the products from the ICS process are minerals that are already abundant in the environment. It merely accelerates natural processes, thereby countering mankind’s acceleration of naturally occurring processes that result in carbon dioxide emissions: the burning of organic matter.
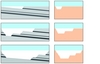
The ICS process draws on knowledge and processes that are familiar to many industries. The capture of carbon dioxide into an ammonia-rich solution dates back to the 19th century, to the venerable Solvay process, still used for the manufacture of soda ash and bicarbonate of soda from salt and flue gases. The quarrying and subsequent treatment of serpentinite or other ultramafic rock involves handling large tonnages, but nothing that is beyond the experience of major mining operations. While it indeed takes around six tonnes of serpentinite or equivalent to store the carbon dioxide from burning a tonne of black coal, on a volume basis, this rock in situ occupies around 2.5 cubic metres. Open-cut coal mining often involves having to remove at least 5 cubic metres of overburden and other rock to access each tonne of coal (Figure 2).
The equipment for separating the carbonated rock from the capture solution can be found in any large hydrometallurgical operation, although the final product both weighs more (because of the added carbon dioxide) and occupies a greater volume than the precursor ultramafic rock. There will, therefore, be a surplus after quarries have been back-filled, but this surplus can be put to other uses including the rehabilitation of old open-cut coal mining areas. The final repositories, whether these are in old quarry or mine voids, or in purpose-built dams, will of course need to be engineered competently, but they will be free from concerns about toxic chemicals and acid mine drainage that can be a feature of, for example, tailings from gold and base-metal mining operations, and even from coal preparation plants.
The inert nature of the carbonated rock and the fact that the ICS process needs only familiar plant and equipment suggest a quite rapid path to its commercialisation and, critically, the potential for worldwide deployment, to the extent where serious inroads into carbon dioxide emissions from coal-fired power stations may be expected within a couple of decades.
Byproduct credits
Significantly, the ultramafic rocks used in the ICS process tend also to host many important base metals including nickel, chromium, platinum-group metals, copper-gold, and iron. The ICS process would convert many of these metals to more readily recoverable forms, eg iron to magnetite, and nickel and copper to ammines in solution.
Their heavy metal content leads to a common surface indicator for ultramafic rocks: natural vegetation communities hosted in soils formed on them tend to be impoverished, trees tend to be few and stunted even in high rainfall areas, while grasses and other plants are hardy and slow-growing. Such areas are well described by the term ‘serpentine barrens’. It follows that the recovery of these metals will yield, as a further bonus, more productive soils – as a side effect of emplacement of the carbonated product of the ICS process.
The potential revenue from the recovery of such metals as byproducts could offset partially, and at times fully, the cost of carbon capture and storage. By way of example, a typical serpentinite may contain 10 per cent magnetite and 0.2 per cent nickel-equivalent (including cobalt). Since to capture one tonne of carbon dioxide it takes three tonnes of serpentinite, with reasonable assumptions for recoveries we can assume byproduct production of 250 kg of magnetite and 4 kg of nickel per tonne of carbon dioxide captured, which at US$40/tonne and US$10/kilogram respectively, should yield gross revenues of US$50/tonne of carbon dioxide captured, ie, a sum sufficient to offset fully, the cost of capturing and storing that quantity of CO2. While the additional costs involved in recovering these byproducts in marketable form would need to be included in any particular total project cost structure, these numbers do indicate the significance of likely base-metal credits. They promise to have a major, positive impact on the economics of the first wave of installations of the ICS process, when such revenues would be needed most.